Stellar Market Research examines the growth rate of the Hernia Mesh Devices Market during the forecast period 2025-2032
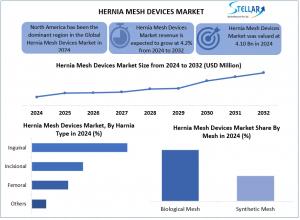
Hernia Mesh Devices Market was valued at USD 4.10 bn in 2024 and is expected to reach USD 5.70 bn by 2032, at a CAGR of 4.2% during the forecast period.
Reinforcing recovery with innovation, hernia mesh devices lead the way in modern surgical solutions.”
ORLANDO, FL, UNITED STATES, June 13, 2025 /EINPresswire.com/ -- The Global Hernia Mesh Devices Market is predicted to grow at a rate of around 4.2% during the forecast period. The Hernia Mesh Devices Market was valued at USD 4.10 billion in 2024 and is projected to reach USD 5.70 billion by 2032. The Hernia Mesh Devices Market is driven by the increasing incidence of hernia cases, advancements in mesh technology, rising number of laparoscopic procedures, growing geriatric population, better access to healthcare, expanded insurance coverage, and clinical awareness are leading to higher demand for minimally invasive (and effective) hernia repair products that are easy for patients.— Dharati Raut
Hernia Mesh Devices Market Overview
Hernia Mesh Devices Market is rising as hernia cases go up, older people live longer, and many more use less painful ways to do surgery. These devices are made from man-made or natural materials. They make the weak spot in the tissue stronger when the hernia is fixed. New tech, good pay, and new ways to do surgeries make more people want these devices. Companies named Medtronic, BD, Ethicon, and Gore are big in the field. Product recalls on some devices and the high price of the devices make it hard in some ways. New places in the world want these devices. New tech and new types of meshes that break down after a time, or are made in a new way, can help the industry grow. The market is split by the kind of material, the kind of surgery, and the kind of hernia. The market will grow much soon in Areas that have good health care and many surgeries done now, and in ones that are rich now, but will grow in riches in the future.
To know the most attractive segments, click here for a free sample of the report: https://www.stellarmr.com/report/req_sample/hernia-mesh-devices-market/2666
Hernia Mesh Devices Market Dynamics
Drivers
Rising Hernia Incidence
The increase in hernias worldwide, exacerbated by aging populations, weight gain, and lifestyle, is providing a tremendous growth opportunity for surgical mesh devices. More than 13 million new hernias are reported globally every year, and mesh repairs are favoured due to lower recurrence rates. While the market is experiencing rapid growth due to innovations, such as absorbable mesh and drug-embedded mesh, as well as food and drug administration (FDA) approvals, it is clear that a growth trajectory remains as the hemodynamics for healthcare continues to improve in most regions. North America maintains the majority of the market, while the Asia-Pacific market in particular is experiencing rapid growth due to more people having access to health services and increasing surgical requests.
Growth in Minimally Invasive Surgeries
The increase in minimally invasive surgeries, especially laparoscopic and robotic-assisted hernia repairs, has increased demand for sophisticated mesh devices. Patients prefer minimally invasive surgery to non-minimally invasive surgery, particularly for a hernia repair, since these repairs are quick, the patient has less pain, and fewer complications. Consumer innovation and adoption, such as self-fixating and 3D meshes, contribute to the shift to surgical interventions. Robotic surgeries are expected to increase around the world, and advanced devices and systems continue their adoption and use by healthcare systems, indicating that the hernia mesh devices market regionally across the globe will continue to grow.
Clinical Guidelines and Surgeon Education
Updated clinical guidelines and surgeon training are driving the adoption of hernia mesh over sutures due to better long-term outcomes and reduced recurrence. Organizations like SAGES and EHS recommend mesh-based repairs. Training programs such as FLS and Medtronic’s hernia courses enhance surgical proficiency. Recent developments, including updated HerniaSurge guidelines and research on mesh materials, reinforce best practices and support the growing use of mesh in hernia surgeries worldwide.
Advancements in Mesh Technology
Advancements in hernia mesh technology, like lightweight, absorbable, composite, and 3D meshes, have enhanced surgical outcomes and patient comfort by reducing infection, adhesion, and recurrence risks. Innovations such as drug-eluting and anti-adhesive meshes, as well as integration with robotic surgery, are revolutionizing hernia repair. Recent launches like Phasix fully absorbable mesh and sensor-enabled designs further strengthen this shift toward safer, more effective, and personalized surgical solutions.
Restrains
Limited Reimbursement in Developing Regions
In developing regions, the lack of good cover for insurance and poor payback by the state makes it hard for people to get the best hernia mesh tools. The high price at the end of the pay line means people have to wait to get their operations, or they might not get them at all, making things worse for them. Things like cheap mesh that can be used for many things and trips to help those who need it (like Operation Hernia) try to fix this. But, to make things better on a wider scale and to get more money to help these systems that do not have much to spend, much more change needs to happen.
Innovations and Developments
Technological innovation is a key factor propelling the Hernia Mesh Devices Market forward. Notable advancements include:
Lightweight and Large-Pore Meshes: Lightweight and big holes in the mesh make it less likely to make the body have a bad response. It also cuts down the pain after the fix. These meshes let the body grow new tissue into them. They bend and move with the body. They are easy to put in. These things make them good for fixing hernias with the help of a scope. They cut down on the pain that stays for a long time and make the fix better.
3D Meshes and Anatomically Contoured Designs: 3D meshes and anatomically contoured designs are pre-shaped to match the body's natural anatomy, minimizing the need for surgical adjustments. They enhance patient comfort, reduce operative time, and improve success rates in hernia repair procedures.
To know the most attractive segments, click here for a free sample of the report: https://www.stellarmr.com/report/req_sample/hernia-mesh-devices-market/2666
Hernia Mesh Devices Market Segmentation
By Hernia
By Hernia, the Hernia Mesh Devices Market is further segmented into Inguinal, Incisional, Femoral, and Others. Among which, Inguinal hernia is the main part of the hernia mesh devices market, making up more than 70% of all cases of stomach hernia. This type of hernia hits about 27% of men and 3% of women around the world. Every year, in the U.S. alone, there are more than 800,000 surgeries to fix inguinal hernia. The big number of cases, good results from surgery, and the fact that doctors like to use mesh for a kind of fixing called laparoscopic all help this kind of hernia to be at the top of the list.
Hernia Mesh Devices Market Regional Analysis
North America: North America dominates the hernia mesh devices market, performing over 1 million hernia surgeries annually, including 800,000 inguinal repairs. Strong reimbursement systems, advanced surgical infrastructure, and the presence of major players like Medtronic and Gore drive growth. Recent innovations include Medtronic's Hugo robotic system trials and Gore’s collaboration to reduce mesh manufacturing costs through advanced purification technologies.
Europe: Europe ranks second in the hernia mesh devices market due to its aging population, universal healthcare systems, adherence to European Hernia Society guidelines, and strong presence of companies like B. Braun and Molnlycke. Recent NHS robotic surgery adoption boosts regional growth.
Asia-Pacific: Asia-Pacific ranks third owing to its elderly population, more hernia surgeries, more health care buildings, and more people visiting health care. Countries like India, China and Japan make people want new mesh tools.
Hernia Mesh Devices Market Competitive Landscape
The global and regional players in the Hernia Mesh Devices Market concentrate on developing and enhancing their capabilities, resulting in fierce competition. Notable players include:
Johnson & Johnson Services, Inc. (New Brunswick, New Jersey, USA)
Becton, Dickinson and Company (C. R. Bard, Inc.) (Franklin Lakes, New Jersey, USA)
W. L. Gore & Associates, Inc. (Newark, Delaware, USA)
Atrium Medical (Getinge Group) (Hudson, New Hampshire, USA)
LifeCell (Branchburg, New Jersey, USA)
Baxter International Inc. (Deerfield, Illinois, USA)
Cook Group Incorporated (Bloomington, Indiana, USA)
Deep Blue Medical Advances Inc. (Durham, North Carolina, USA)
Integra LifeSciences Holdings Corporation (Princeton, New Jersey, USA)
RTI Surgical (Alachua, Florida, USA)
TELA Bio (Malvern, Pennsylvania, USA)
B. Braun Melsungen AG (Melsungen, Germany)
Summary
The Global Hernia Mesh Devices Market is projected to grow at a CAGR of 4.2%, reaching USD 5.70 billion by 2032 from USD 4.10 billion in 2024. Growth is driven by rising hernia cases, technological advancements (like absorbable and 3D meshes), and increasing adoption of minimally invasive surgeries. Key players include Medtronic, BD, Ethicon, Gore, and B. Braun. The market faces hurdles such as high device costs and limited insurance in developing regions. Inguinal hernia dominates by type, accounting for over 70% of cases. Regionally, North America leads due to robust infrastructure and high surgery rates, followed by Europe and Asia-Pacific, where access to healthcare is improving. Innovations like drug-eluting meshes and robotic surgery integration continue to boost demand. The market is segmented by hernia type, surgery type, and mesh material.
Related Reports:
At Home Testing Market: https://www.stellarmr.com/report/at-home-testing-market/2397
Sperm Bank Market: https://www.stellarmr.com/report/sperm-bank-market/2395
Nutrigenomics Market: https://www.stellarmr.com/report/nutrigenomics-market/2392
Dental Chair Market: https://www.stellarmr.com/report/dental-chair-market/2389
Gamma Knife Market: https://www.stellarmr.com/report/gamma-knife-market/2382
About Stellar Market Research:
Stellar Market Research is a multifaceted market research and consulting company with professionals from several industries. Some of the industries we cover include medical devices, pharmaceutical manufacturers, science and engineering, electronic components, industrial equipment, technology and communication, cars and automobiles, chemical products and substances, general merchandise, beverages, personal care, and automated systems. To mention a few, we provide market-verified industry estimations, technical trend analysis, crucial market research, strategic advice, competition analysis, production and demand analysis, and client impact studies.
Contact Stellar Market Research:
S.no.8, h.no. 4-8 Pl.7/4, Kothrud,
Pinnac Memories Fl. No. 3, Kothrud, Pune,
Pune, Maharashtra, 411029
sales@stellarmr.com
Lumawant Godage
Stellar Market Research
+91 9607365656
email us here
Visit us on social media:
LinkedIn
Instagram
X
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.